
Learn more on how to publish your next paper with hindawi Learn more on how to publish your next paper with hindawi
Researchers are now studying stem cells to see if they could help treat a variety of conditions that impact different body systems and parts.
T cell gene therapy. It is worth considering how biosimilar competition will play out with fda approved cell and gene therapies. Viral escape mutants are likely to develop, the tcr repertoire of resistant cells may be limited, and t cells represent only one of the reservoirs of infected cells in the body (e.g. The cells used to produce cell therapy products include hematopoietic stem cells and adult.
6 gene therapy clinical trials are currently evaluating treatments for severe combined immunodeficiency, eye diseases, hiv, sickle cell anemia, cystic fibrosis, congestive heart failure, hemophilia, cancer, and various other. So far some of the biggest strides in this new field of medicine have been in oncology. Learn more on how to publish your next paper with hindawi
Together cell and gene therapy could change the way we approach defeating a disease. What works for antibody biologics should also work for cell/gene therapy biologics. Peer reviewed, online & oa.
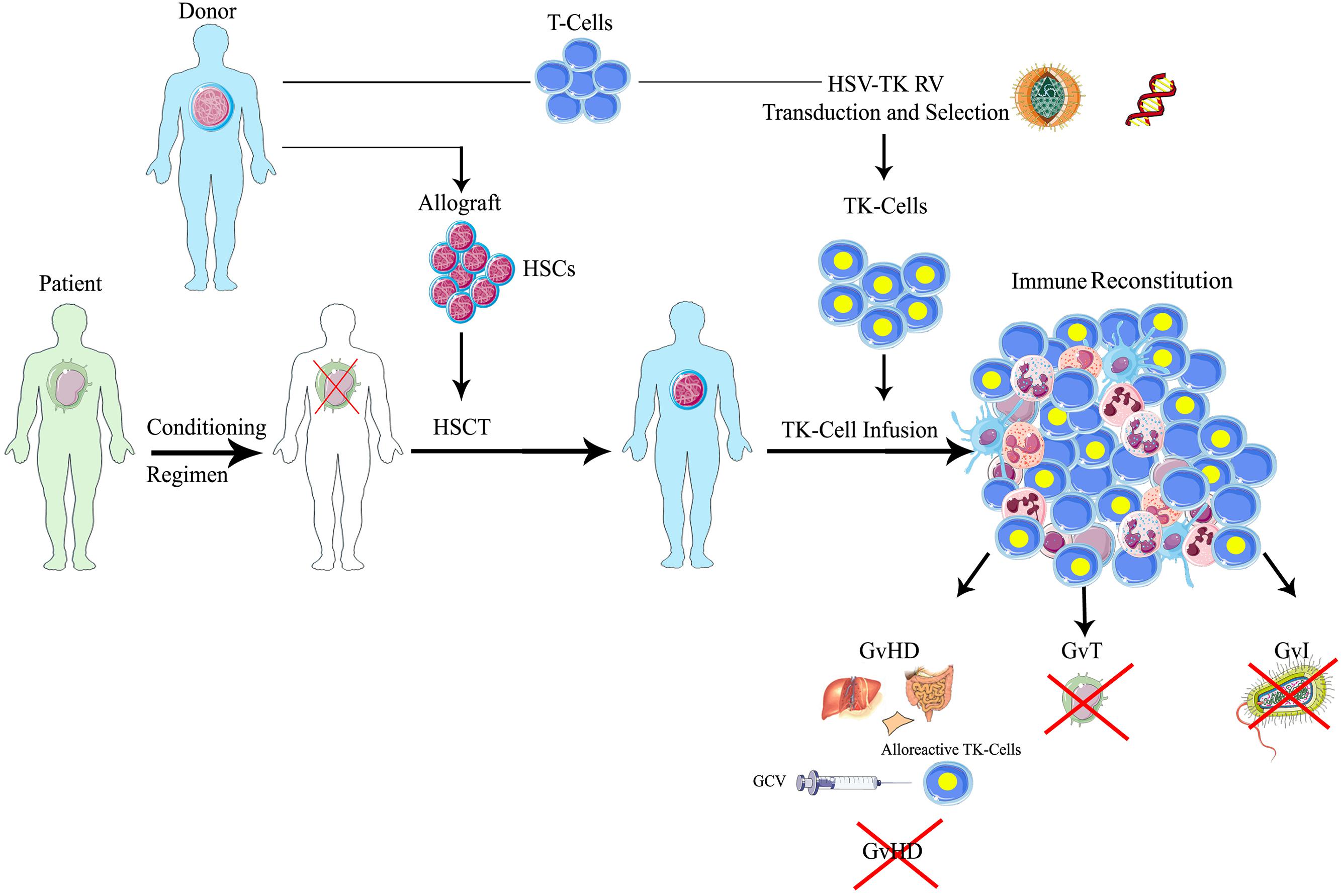
The use of genetically modified t cells (t lymphocytes) to target cancer is a promising approach, especially for cancers that are difficult to treat using traditional methods. In the past year, a number of human gene therapy trials involving the adoptive transfer of genetically modified t lymphocytes have been reported. Food and drug administration (fda).
The endogenous tcrα and tcrβ chains (orange), the introduced tcrα and tcrβ chains (blue), and mixed dimers of the endogenous tcrα or tcrβ chain paired with the introduced tcrβ or tcrα chain, respectively. Cell and gene content collections. In the past year, a number of human gene therapy trials involving the adoptive transfer of genetically modified t lymphocytes have been reported.
The therapy requires drawing blood from patients and separating out the t cells. T cells modified by tcr gene therapy to express a second pair of tcr chains have the potential to form four unique tcrs on their cell surface, consisting of: Car t cells are considered gene therapy, according to the u.s.
Learn more on how to publish your next paper with hindawi Cell and gene therapy products are grouped together because these technologies are often combined. Researchers are now studying stem cells to see if they could help treat a variety of conditions that impact different body systems and parts.
(1)cell genesys inc, 322 lakeside drive, foster city, ca 94404, usa. Peer reviewed, online & oa. Enabling all the parts to work together and get to the right places requires other biological tools, some of which are being developed by uk companies.
Haematopoietic stem cell transplantation and, in specific candidate diseases, haematopoietic stem cell gene therapy has been the only definitive treatment option so far. Hege km (1), roberts mr. Cell therapy products include immunotherapies, cancer vaccines, and other types of both autologous and allogeneic cells designed to treat different conditions.
The list of conditions that stem cell therapy could help treat may be endless, including conditions such as alzheimer’s disease, heart disease, diabetes, and more. In car t cell therapy, a type of lymphocyte called t cells, which play a central role in human immune system, are extracted from each patient and then genetically engineered to produce a special protein called a chimeric antigen receptor (car).