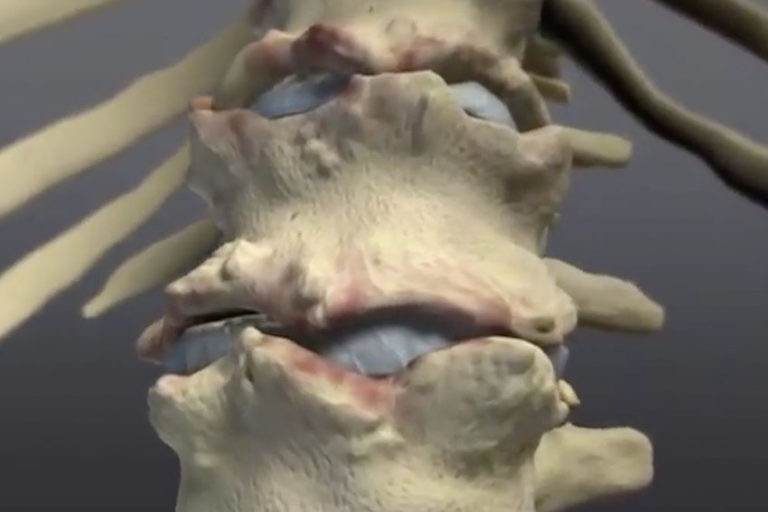
Ankylosing spondylitis (as) is a chronic autoimmune disease that primarily affects the spinal joints, but large joints, such as the hips and. In severe cases, this may cause the bones in the spine to grow together, which can lead to a rigid spine that is difficult to bend.
In severe cases, this may cause the bones in the spine to grow together, which can lead to a rigid spine that is difficult to bend.
Injection for ankylosing spondylitis. The recommended dose is 160 mg by subcutaneous injection (two 80 mg injections) at week 0, followed by 80 mg every 4 weeks. At that point, your doctor may prescribe: Administer cosentyx with or without a loading dosage by subcutaneous injection.
•if a patient continues to have active ankylosing spondylitis, consider a dosage of 300 mg every 4 weeks. Your doctor can inject steroids into the joints, including. The primary end point was a composite of improvements in measures of morning stiffness, spinal pain, functioning, the patient�s global assessment of disease activity, and joint.
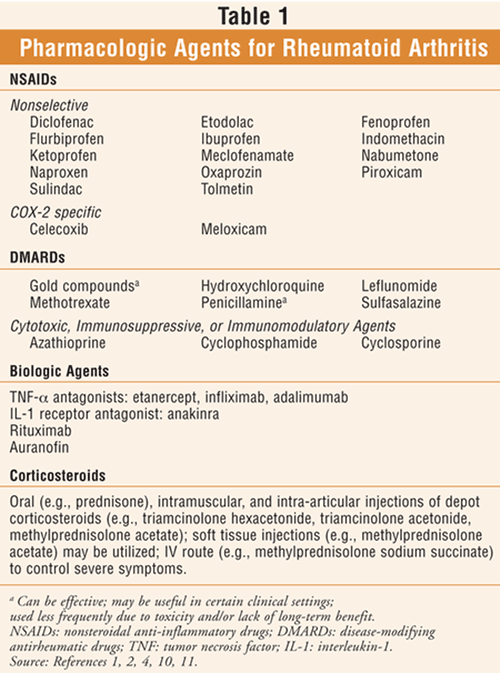
They’re used to help reduce: Over time, this inflammation in the joints and tissues of the spine can cause stiffness. Biologics for ankylosing spondylitis aren’t available in pill form.
65% of reviewers reported a positive effect, while 26% reported a negative effect. Food and drug administration (fda) has approved the supplemental new drug application (snda) for xeljanz ® / xeljanz ® xr. In august 2019 the fda approved eli lilly’s taltz (ixekizumab) injection for the treatment of adults with active ankylosing spondylitis (as).
Ixekizumab is an interleukin inhibitor. Kristen has had ankylosing spondylitis (as), a type of inflammatory arthritis that causes debilitating back and hip pain, as well as other symptoms, for more than 20 years. This review discusses the data that supports use of etanercept in the treatment of ankylosing spondylitis.
Cosentyx (secukinumab) is a prescription drug used to treat plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis. They must be given under the skin, either through an intravenous (iv. The fda has approved xeljanz for the treatment of adults with active ankylosing spondylitis who have had an inadequate response, or intolerance to, tnf inhibitors, according to a pfizer press release.
Ankylosing spondylitis (as) is a disease characterised by inflammation of multiple joint structures, frequently resulting in fusion of the bones and having an affinity for the backbone and skull. Cosentyx (secukinumab), which was approved for as and psa in january of 2016, and taltz (ixekizumab) approved for psa in december of 2017, and for as in august of 2019. Simponi has an average rating of 6.7 out of 10 from a total of 23 ratings for the treatment of ankylosing spondylitis.
Ixekizumab (taltz), was recently approved by the fda for this condition. Most people develop some disabilities due to spinal problems, but can lead normal productive lives. People with active anklyosing spondylitis (as) now have a new treatment option to consider:
Intraarticular sacroiliac corticosteroid injections in ankylosing spondylitis. Ankylosing spondylitis (as) is a chronic autoimmune disease that primarily affects the spinal joints, but large joints, such as the hips and. • with a loading dosage is 150 mg at weeks 0, 1, 2, 3, and 4 and.
Biologics are administered by infusion or injection. In severe cases, this may cause the bones in the spine to grow together, which can lead to a rigid spine that is difficult to bend. Aulfasalazine ( azulfidine ), a tablet to control pain and swelling in smaller joints.
She currently takes the biologic infliximab (remicade), which affects immune system function. Fda approves pfizer’s xeljanz® (tofacitinib) for the treatment of active ankylosing spondylitis. Ankylosing spondylitis is a type of arthritis that causes inflammation in certain parts of the spine.
She has lung and kidney damage (only one kidney left) as a result of extensive. User reviews for simponi to treat ankylosing spondylitis. You can take tnf inhibitors either through a subcutaneous injection or an intravenous (iv) line.
Pfe) announced today that the u.s. Intraperitoneal injection of human fetal cartilage proteoglycan (depleted of chondroitin sulfate) in freund�s complete or incomplete adjuvant induces a chronic erosive polyarthritis and spondylitis in all female balb/c mice.