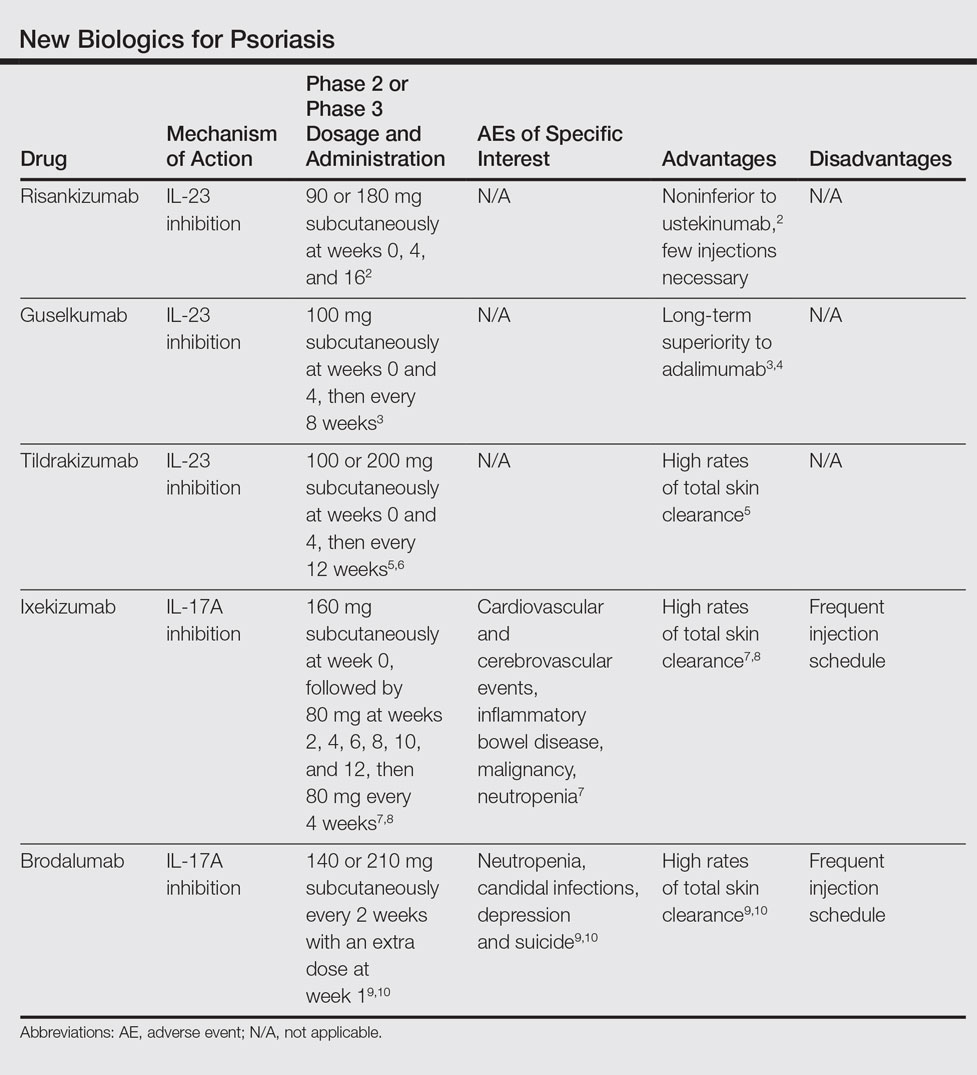
In the initial efficacy trials examining the use of ixekizumab for plaque psoriasis, new presentations of ibd were not found to be a frequent adverse effect in the experimental groups [ 4 ]. The efficacy and safety data from key phase iii clinical trials are reviewed here.
In the initial efficacy trials examining the use of ixekizumab for plaque psoriasis, new presentations of ibd were not found to be a frequent adverse effect in the experimental groups [ 4 ].
Il 17 psoriasis drugs. Fda approved injectable biologics, secukinumab and ixekizumab. The indications for which these therapeutics are most advanced — that is, have reached or are close to regulatory approval — are psoriasis, psoriatic arthritis and ankylosing spondylitis. Interleukin inhibitors global market report 2021:
There is no cure for psoriasis. Psoriasis is a frequent chronic inflammatory skin disease, nowadays considered a major global health problem. Bartlett and million discuss the key agents in the pipeline, several of which are.
While these drugs are very effective, drugs that. In the initial efficacy trials examining the use of ixekizumab for plaque psoriasis, new presentations of ibd were not found to be a frequent adverse effect in the experimental groups [ 4 ]. These therapies represent a major improvement of the way in which psoriasis is managed, since they show an unprecedented efficacy on skin.
The efficacy and safety data from key phase iii clinical trials are reviewed here. 68 the recommended dosing schedule of brodalumab for adults is 210 mg at weeks 0, 1, and 2, followed by 210 mg every 2 weeks.
