Csl behring and uniqure n.v.�s (nasdaq: We believe etranacogene dezaparvovec may be the first gene therapy to provide durable, functionally curative benefits to nearly all patients.
In the last decade, enormous progress has been made in the development of gene therapy for hemophilia a and b.
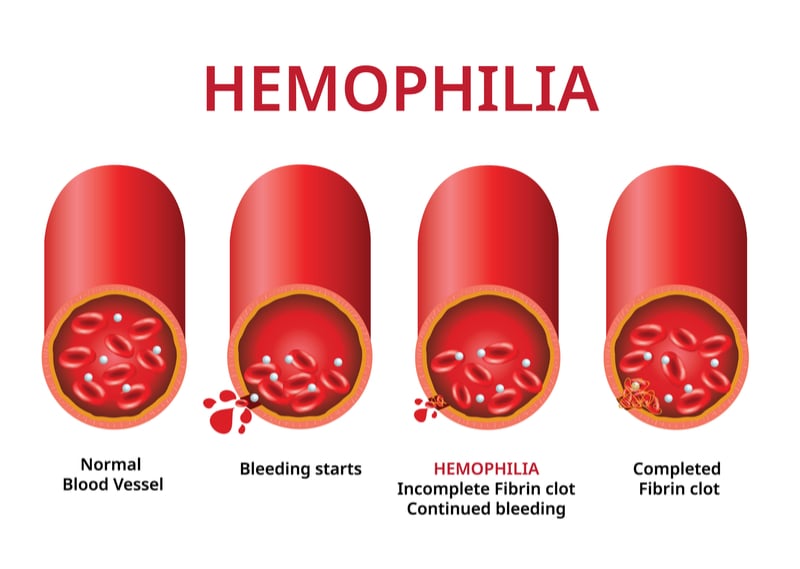
Hemophilia b gene therapy. Gene therapy has the potential to maintain therapeutic blood clotting factor ix (fix) levels in patients with hemophilia b by delivering a functional human f9 gene into liver cells. The trial sponsored by university college, london (ucl) and st. Hemophilia is a genetic disease that prevents blood from clotting properly leading to prolonged internal and external bleeding.
Gene therapy offers the potential for a cure for patients with hemophilia by establishing continuous endogenous expression of factor viii or factor ix (fix) following transfer of a functional gene to replace the hemophilic patient’s own defective gene. We believe etranacogene dezaparvovec may be the first gene therapy to provide durable, functionally curative benefits to nearly all patients. The annualized bleeding rate was 11.1 events per year before.
After decades of development, gene therapy appears to be nearing the clinic for hemophilia b, with hemophilia a not far behind. The effects of an experimental gene therapy for severe hemophilia appear to be holding up relatively well, according to clinical trial data released thursday by the dutch biotech uniqure.; This is one of the most exciting times in the course of hemophilia.
This therapy is currently being investigated in a phase ii clinical trial (nct02484092). The trial sponsored by university college, london (ucl) and st. Csl behring and uniqure n.v.�s (nasdaq:
Listed are the name of the gene therapy product, some of the vector details (eg, aav vector serotype, form of fix cdna, and type of vector), the phase of clinical trial development and the industry sponsor of the study. In the last decade, enormous progress has been made in the development of gene therapy for hemophilia a and b. The trial is also the first to include patients with certain immune system markers and found that they did not appear to.
Uniqure and baxalta are both testing gene therapies for hemophilia b. It’s not available yet, but after 20 years in. The phase 3 program was initiated following the transfer of the responsibility for spark therapeutics’ hemophilia b gene therapy program to pfizer.
Gene therapy has the potential to maintain. Hemophilia is amenable to gene therapy because both the a and b types are the result of a variant in a single gene. The trial enrolled patients with the less common, b form of the bleeding disease, and tracked 53 of them for a total of 18 months.
Hemophilia b is caused by mutations in the f9 gene, which provides instructions for making the clotting protein factor ix, known as fix. Hemophilia b clinical gene therapy trials (september 2020).