
After this reengineering, the t cells. Despite encouraging results in phase 1 clinical trials targeting glioblastoma (gbm), these efforts have not been as successful as those targeting hematological malignancies.
Synthetic biology approaches for cellular engineering provide a broadly expanded set of tools to program immune cells for enhanced function.
Chimeric antigen receptor t cell. These receptors are synthetic molecules, they don�t exist naturally, explained carl june, m.d., of the university of pennsylvania abramson cancer center, during a recent presentation on car t cells at the. After this reengineering, the t cells. Effective adoptive t cell therapy (act) comprises the killing of cancer cells through the therapeutic use of transferred t cells.
Next, using a disarmed virus, the t cells are genetically engineered to produce receptors on their surface called chimeric antigen receptors, or cars. Yet the use of car t cells is limited by potentially severe toxicities. Synthetic biology approaches for cellular engineering provide a broadly expanded set of tools to program immune cells for enhanced function.
Chimeric antigen receptor (car) t cells can produce durable remissions in hematologic malignancies that are not responsive to standard therapies. The cells are sent to a The cause of neurotoxicity is incompletely understood, and its unpredictability is a reason for prolonged hospitalization after.
Glioblastoma multiforme (gbm) is the most common malignant brain cancer that invades normal brain tissue and impedes surgical eradication, resulting in. Despite encouraging results in phase 1 clinical trials targeting glioblastoma (gbm), these efforts have not been as successful as those targeting hematological malignancies. Category iii cpt code) 0539t.
Car t cells are then expanded for clinical use and infused back into the patient�s body to attack and. Adoptive transfer of chimeric antigen receptor (car) t cells is a powerful targeted immunotherapeutic technique. One of the main act approaches is chimeric antigen receptor (car) t cell therapy.
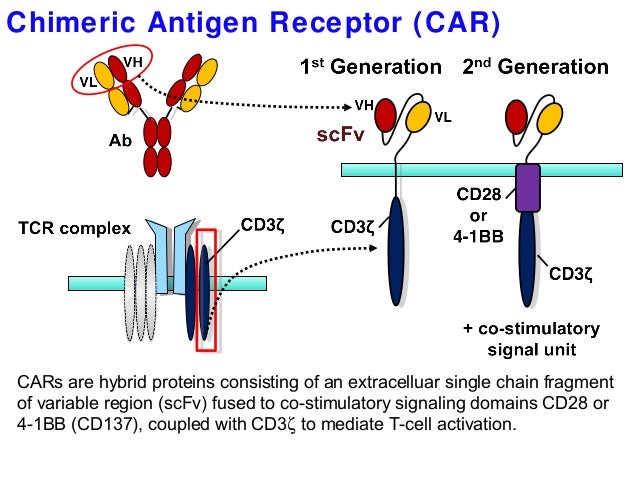
In this strategy, a patient�s own t cells are genetically engineered to express a synthetic receptor that binds a tumor antigen. This form of immunotherapy has transformed cancer care over the past decade ( 1 ). 31 the chimeric antigen receptor consists of an extracellular domain, which engages the target antigen, and an intracellular signal transduction domain.
It is coupled to the car structure. Recently, chimeric antigen receptor (car) technology has revolutionized cancer therapy. Charging the t cells to fight.
The t cells are sent to a laboratory or a drug manufacturing facility where they are genetically engineered, by introducing dna into them, to produce chimeric antigen receptors (cars) on the surface of the cells. Chimeric antigen receptor (car) t cells were recently approved by the food and drug administration (fda) and are poised to enter the practice of medicine for the treatment of leukemia and lymphoma (see video). T lymphocytes engineered to express chimeric antigen receptors (cars) can selectively target and eliminate cancer cells while exerting limited cytotoxicity against normal tissues.