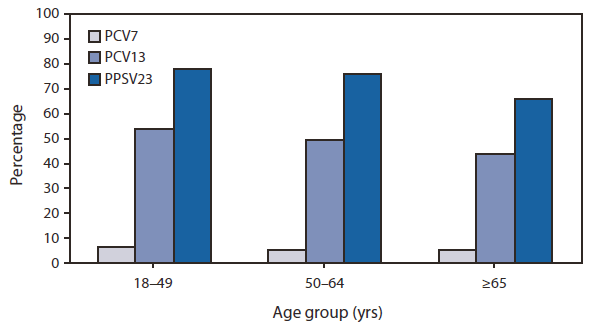
Prevnar® 13 should be offered a single dose of prevnar® 13 if they present prior to their 5. Why is prevnar 13 not approved by the cdc for asthmatics?
Sales exploded in 2015 after the cdc�s recommendation, and although insurance will still cover the pricey vaccine, sales would drop if fewer adults feel like they need to get it.
Cdc prevnar 13 guidelines. Cdc recommends pcv13 for all children younger than 2 years old and people 2 years or older with certain medical conditions. 12 to less than 24 months of age: Children younger than 2 years old.
Prevnar 13® is a vaccine approved for adults 18 years of age and older for the. Cdc pneumococcal vaccination recommendations for adults pcv13 & ppsv23 key concepts pcv13: Children who miss their shots or start the series later should still get the vaccine.
When both are indicated, pcv13 should be given before ppsv23 whenever possible. When the decision to administer prevnar 13® is made, it should be given first in series with pneumovax 23® at least one year apart. The cdc’s new recommendation involves pcv13, “the changes are that everybody over the age of 65 should still get the pneumovax 23 but the prevnar 13 is a discussion to have with your physician.
Prevnar® 13 should be offered a single dose of prevnar® 13 if they present prior to their 5. Adults 65 years or older also can discuss and decide, with their clinician, to get pcv13. Sales exploded in 2015 after the cdc�s recommendation, and although insurance will still cover the pricey vaccine, sales would drop if fewer adults feel like they need to get it.
(nyse:pfe) announced today that the centers for disease control and prevention’s (cdc) advisory committee on immunization. Cdc recommends pcv13 for all infants as a series of 4 doses. Prevnar 13® is indicated for active immunization for the prevention of pneumonia and invasive disease caused by streptococcus pneumoniae serotypes 1, 3, 4, 5, 6a, 6b, 7f, 9v, 14, 18c, 19a, 19f, and 23f in adults 18 years of age and older
A o sickle cell disease and other hemoglobinopathies Prevnar 20 is designed to protect against more strains of the pneumococcal bacteria that can cause lung, ear, sinus and blood infections compared to pfizer’s older prevnar 13, which is widely used and routinely given to children under the age of 2. The recommendations are being reviewed by the cdc director and u.s.
Learn more below about which pneumococcal vaccines cdc recommends by age group and medical condition. 1 pneumovax 23® is routinely recommended in adults 65 and older. I cannot speak for the centers for disease control and prevention (cdc) but their recommendations are generally conservative and whenever possible.
Pcv13 should be given before ppsv23, when possible, due to a better immunologic response. Why is prevnar 13 not approved by the cdc for asthmatics? Department of health and human services, and, if approved, they will be published in the morbidity and mortality weekly report.
Without this recommendation, insurance will not cover the cost of the prevnar vaccine but they will cover cost of the pneumovax 23. Give 1 dose at 2 months, 4 months, 6 months, and 12 through 15 months. Cdc used to recommend routine use of two pneumococcal vaccines for all adults aged 65 years or older.
Previously, according to a pfizer press release, no pneumococcal conjugate vaccine has been routinely recommended for certain risk populations aged 19 to 64. If either vaccine is inadvertently given earlier than the For children who have underlying medical conditions, a supplemental pcv13 dose is recommended through age 71 months.
Aged 65 and older with chronic heart disease (chd)