11 first, the process involves using leukapheresis to remove blood from the patient’s body, separate the leukocytes, and return the remainder of the blood to the circulation. The majority of car t cells had a central and effector memory phenotype.
11 first, the process involves using leukapheresis to remove blood from the patient’s body, separate the leukocytes, and return the remainder of the blood to the circulation.
Car t cells review. 12 after a sufficient number of leukocytes have. Leukemia is the most common cancer subtype and accounts for 57.4% (n = 120) of disease indications. Early studies in animal models have suggested that this dual targeting may prevent antigen loss.
Thus, car t cells are arguably one of the first successful examples of synthetic biology and personalized cellular cancer therapy to become commercially available. Car t cells were measurable up to 6 months with a mean persistence of 70.5 ± 14.85 days (follow up ranging from 28 days to 1 year). (1) better safety, such as a.
However, this success has yet to be extrapolated to solid tumors, and the reasons for this are being actively investigated. The production of car t cells requires several carefully performed steps, and quality control testing is performed throughout the entire protocol. Only the choice of initial blood donor is.
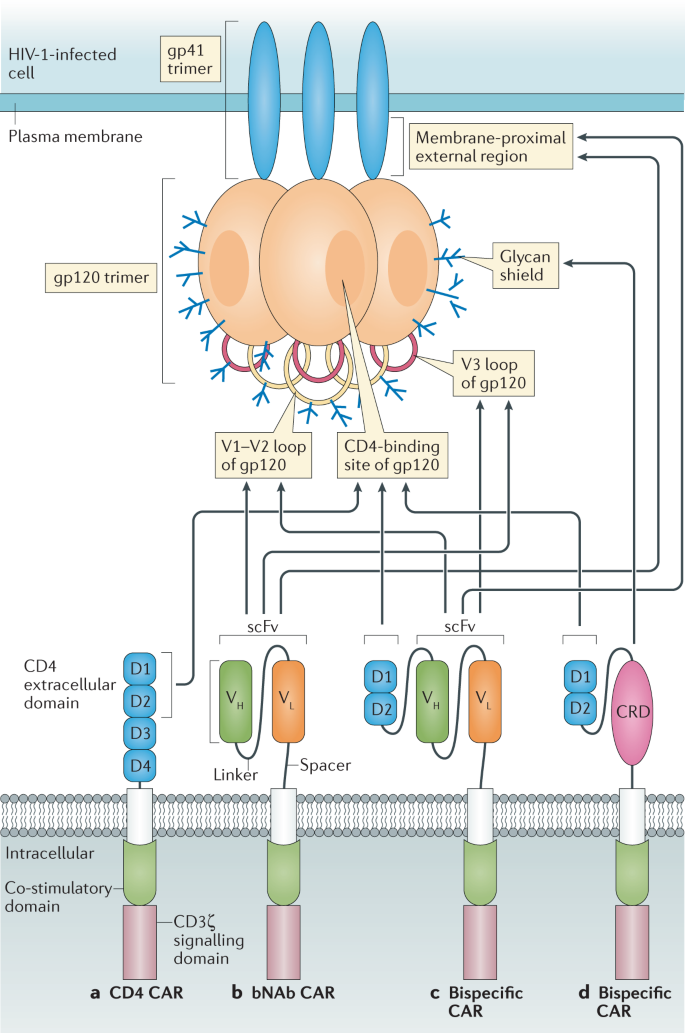
These challenges and the developed strategies to overcome them were reviewed by others. Car t cells specific for aspergillus fumigatus The presence of a set of mechanisms in tumor.
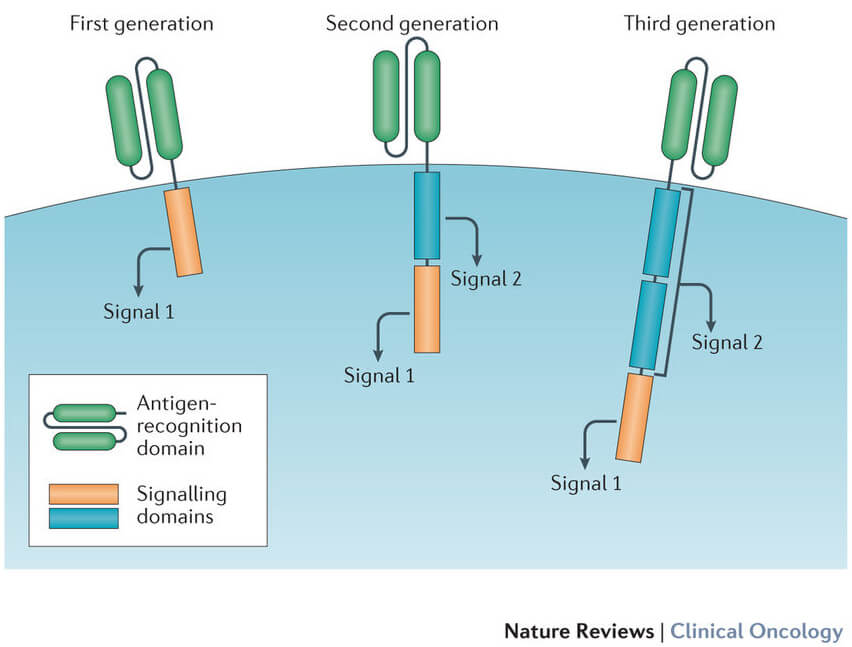
11 first, the process involves using leukapheresis to remove blood from the patient’s body, separate the leukocytes, and return the remainder of the blood to the circulation. This new era of synthetic biology and medicine has been heralded by the recent fda approval of cd19 car t cells for the treatment of all and dlbcl. In this review, we introduce the concept of using car t cells to break immunological tolerance to tumors, highlight several challenges in the field, discuss the utility of biomarkers in the context of predicting.
This has made many realize that the technology might not be as perfect as originally expected. Chop researchers are also testing a car t cell that targets both cd19 and cd123, another antigen commonly found on leukemia cells. We characterize some of the challenges that car t cells have to surmount in the solid tumor microenvironment and new.
No significant difference was observed by integration analyses of the patients pb and cell products. Car t cells are changing the paradigm for cancer therapy. Several conventional treatments and cytotoxic immunotherapies have been developed and made available to the market.
A new era for cancer treatment (review) cancer has recently been identified as the leading cause of mortality worldwide. The majority of car t cells had a central and effector memory phenotype. The manufacturing process is the same in both cases;
Car t cells can cause toxicity by several mechanisms. Further studies on impact of various t cell. The majority of studies used an autologous cell source (85%, n = 17) rather than an allogeneic cell source.
To date, car t cells have demonstrated tremendous success in eradicating hematologic malignancies (e.g., cd19 cars in leukemias).