
Oluwole, mbbs, md, discusses the approvals of tisagenlecleucel and axicabtagene ciloleucel. We developed tisagenlecleucel, and treated the first patients in 2010.
It is approved for children and young adults up to 25 years of age who relapsed or did not respond to therapy for acute lymphoblastic leukemia (all).
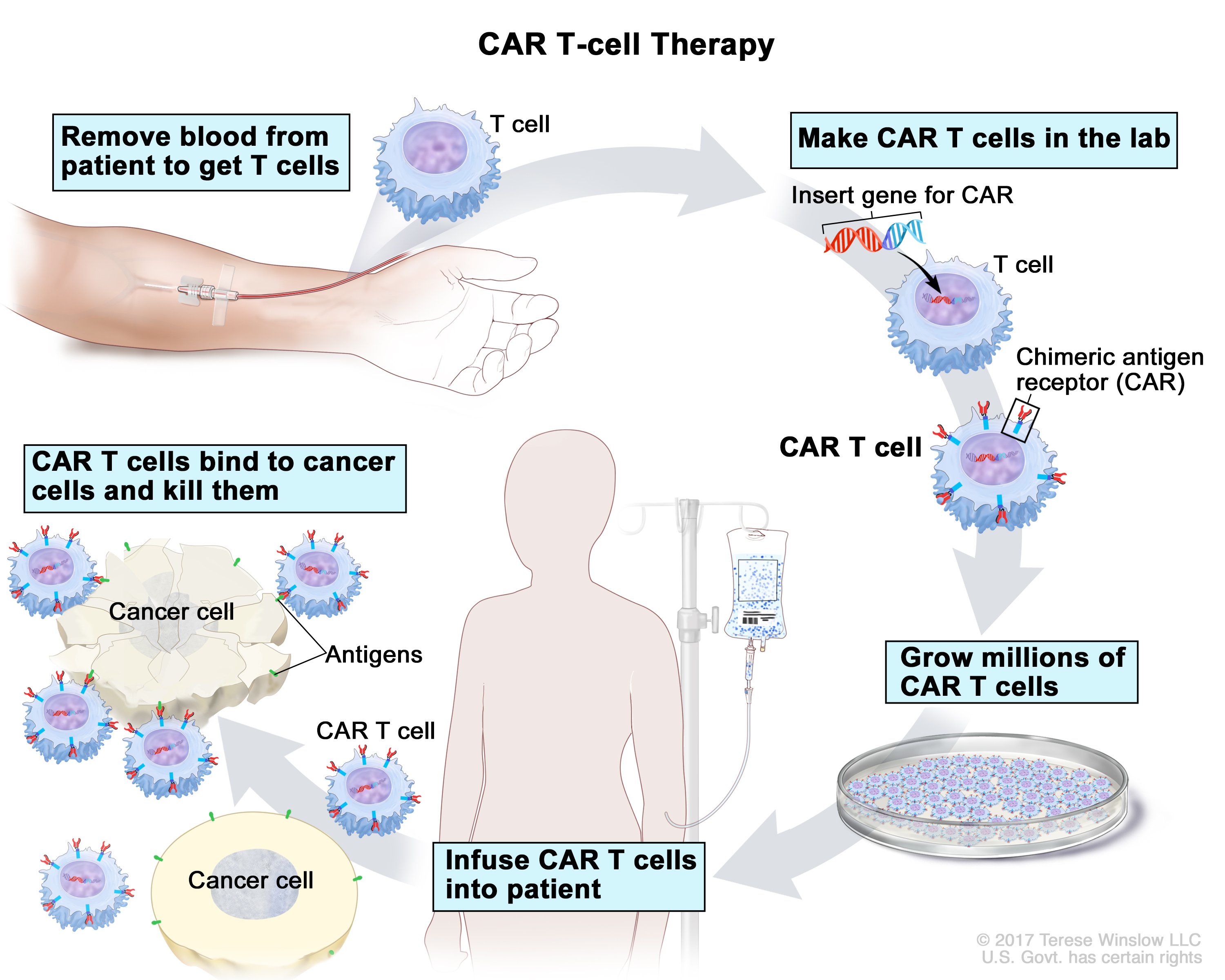
Car t cell therapy leukemia. Hindawi�s academic journals cover a wide range of disciplines. In the process, the modified t cells “recruit” and “educate” additional t cells from the patient’s body to help them fight the cancer. Because the treatment is highly specific to the individual, and requires access to advanced.
Car t cells for acute myeloid leukemia: Kymriah (tisagenlecleucel) is currently approved to treat children and young adults with leukemia. However, the complex procedure for.
Our scientists played several key roles in car t cell therapy development. State of the art and future directions. A promising target the car t cells that have now received fda approval target a molecule on white blood cells called cd19.
Learn more about this and other clinical trials at seattle children�s. Oluwole, mbbs, md, discusses the approvals of tisagenlecleucel and axicabtagene ciloleucel. Patients are evaluated carefully to determine if this therapy is appropriate for.
Acute lymphoblastic leukemia is the most common. Relapse after conventional chemotherapy remains a major problem in patients with myeloid malignancies such as acute myeloid leukemia (aml), and the major cause of death after diagnosis of aml is from relapsed disease. This includes antithymocyte globulin (atg) formulations.
Transforming the treatment of relapsed and refractory disease. In what is being hailed as a watershed moment for both cancer care and biotechnology, the us food and. This novel treatment approach involves collecting t cells from a sample of a patient’s blood, then genetically modifying those cells in a lab to create special structures known as chimeric antigen receptors (cars) on their surface.
Car t cell therapy works by modifying a patient’s t cells to attack leukemia cells. It is approved for children and young adults up to 25 years of age who relapsed or did not respond to therapy for acute lymphoblastic leukemia (all). We developed tisagenlecleucel, and treated the first patients in 2010.
The following year, the fda granted car t cells a breakthrough therapy designation for relapsed or refractory acute lymphoblastic leukemia.