(funded by kite, a gilead company; Submit your paper on the cellular & molecular pathophysiological mechanisms of oxidants.

Yescarta, originally approved in the us in 2017 and in europe in 2018, is indicated for.
Car t cell therapy kite. As the cell therapy leader, kite has more approved car t indications to help more patients than any other company. Since 2000, she has been a lymphoma program/ research nurse coordinating autologous stem cell transplants and in 2015 added car t research trials. Today, we are a leader in engineered t cell therapy, which has changed the paradigm of cancer treatment as one of the biggest breakthroughs in medicine since the introduction of combination chemotherapy more than 60 years ago.
Kite’s singular focus is cell therapy to treat and potentially cure cancer. As the cell therapy leader, kite has more approved car t indications to. Kite is also building a third commercial cell therapy manufacturing facility in frederick county, maryland, which will significantly expand the company’s ability to manufacture car t cell therapies.
For more information on kite, please visit www.kitepharma.com. Patients had disease that had relapsed or was refractory after the receipt of up to five previous. In addition to producing novel car t cell therapies, the clinical manufacturing network is also producing investigational t cell receptor therapies for.
Kite has been at the forefront of cancer immunotherapy since 2009. Tcr therapy is investigational, and its safety and efficacy have not been established. Yescarta, originally approved in the us in 2017 and in europe in 2018, is indicated for.
As a joint venture between shanghai fosun pharmaceutical (group) co., ltd and kite pharma (a gilead company) in u.s., fosun kite is dedicated to the advancement of innovative immune cell therapy and its commercialization to benefit cancer patients in china. Kite’s singular focus is cell therapy to treat and potentially cure cancer. Submit your paper on the cellular & molecular pathophysiological mechanisms of oxidants.
Dedicated to the advancement of innovative immune cell therapies. The decision in juno v kite is not a surprise in light of the recent cafc case law on written description for antibodies, and represents yet another nail in the coffin of functional genus claiming for. (funded by kite, a gilead company;
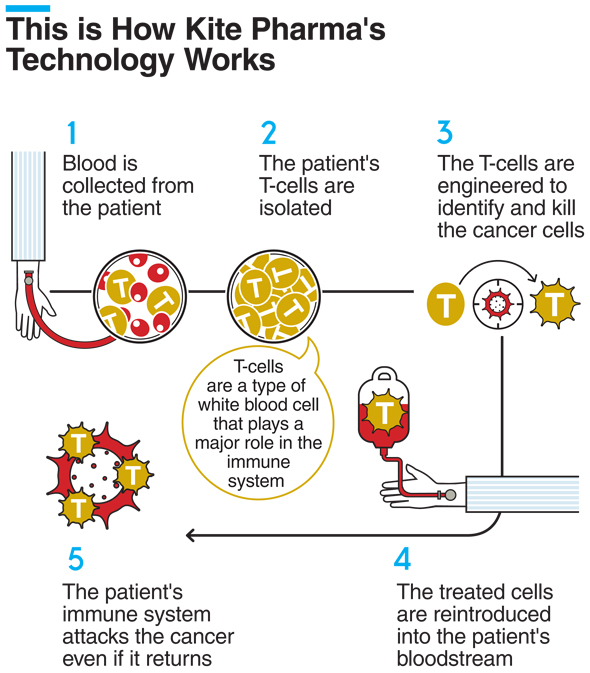
Kite’s singular focus is cell therapy to treat and potentially cure cancer. Then, engineered cells are expanded in the lab and reinfused back into the patient’s blood stream. As the cell therapy leader, kite has more approved car t indications to.
The facility, operated by gilead�s kite unit, will house european production for cell therapy yescarta, which won an ema approval back in.