The hepatitis c virus is identi˜ed. The hepatitis c virus is identi˜ed.
It is developing a treatment of four.
Abbvie hep c treatment. Nhs england’s plan to eliminate hepatitis c in england by 2025 is on track after all aspects of a high court challenge by pharmaceutical company abbvie were dismissed. Designated as a “breakthrough therapy” by the u.s. May be eligible for treatment with mavyret.
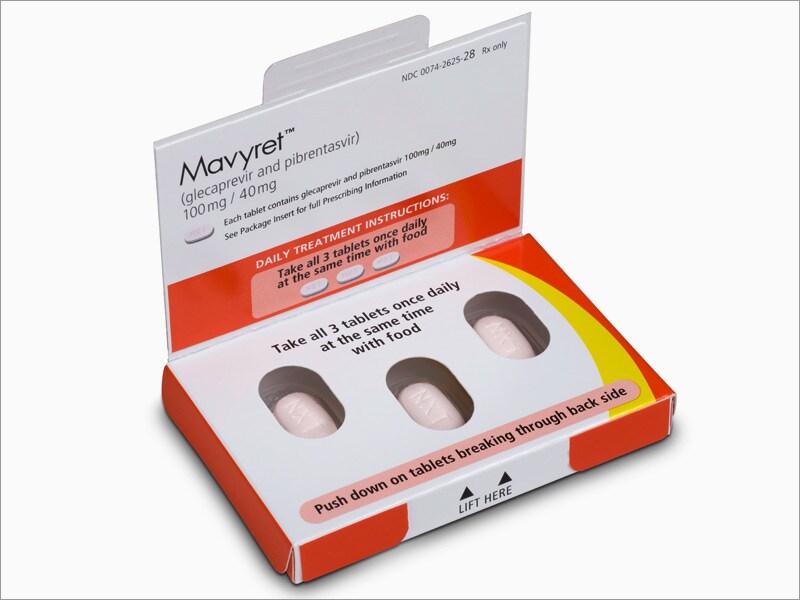
Abbvie’s investigational triple combination hepatitis c therapy cured an average of 98 percent of those with genotype 1b of the virus. Mavyret is a prescription medicine used to treat adults and children 12 years of age and older or weighing at least 99 pounds (45 kilograms) with chronic (lasting a long time) hepatitis c virus (hep c): About 3.4 million americans are living with hep c, according to the drugmaker.
Gilead�s sovaldi is the first in a new wave of hep c treatments that promise better cure rates and a shorter treatment course. Mavyret is a prescription medicine used to treat adults and children 12 years of age and older or weighing at least 99 pounds (45 kilograms) with chronic (lasting a long time) hepatitis c virus (hep c): These are just a few of our efforts to date:
By the time abbvie brought its first drug for hepatitis c to market, in december 2014, it was already too late. Mavyret ( glecaprevir 100 mg and pibrentasvir 40 mg) is taken once daily (three tablets for a total daily dose of glecaprevir 300 mg and pibrentasvir 120 mg) to treat. Provide more treatment for hepatitis c in the community for patients closer to home, by their gps.
The hepatitis c virus is identi˜ed. Ask your doctor if you have any questions about why maviret ® has been prescribed for you. Drugs like abbvie�s mavyret (glecapravir/pibrentasvir) and gilead�s epclusa (sofosbuvir/valpatasvir) essentially cure most patients with the infectious disease in as little as eight weeks of treatment.
Abbvie is not far behind gilead. Eliminating hepatitis c will mean uniting all of us behind a collective effort to overcome the obstacles associated with the disease. Genotypes (gt) 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis
98% of people who take maviret † as directed and who are new to treatment can expect to be cured* of their hepatitis c (hep c), meaning that the hep c virus has been cleared from the body. It is developing a treatment of four. The nhs’s single largest medicines procurement, a deal worth almost £1 billion over five years, was launched in april last year but contract start dates had to be delayed by six months after legal.
Twelve months earlier, gilead sciences had secured u.s. According to abbvie’s release, up to 95% of hep c patients in the u.s. Earlier hepatitis c drugs came in at wholesale costs of $84,000 and $94,000 per treatment course, but competition has pressured net prices of newer entrants.
Abbvie’s regimen is a three pronged attack on the hepatitis c virus: The abbvie hep c information website, www.hepcinfo.co.nz,. The second, harvoni, started at $94,500.
The food and drug administration (fda) recently approved abbvie’s mavyret, the newest treatment for chronic hepatitis c virus infection for people without cirrhosis or with compensated cirrhosis. Gilead’s sovaldi, the first antiviral for hepatitis c, launched at $84,000 for a course of treatment; The food and drug administration said last week abbvie inc had identified cases of hepatic decompensation and liver failure in.
Genotypes (gt) 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis. The program seeks to educate current doctors who are looking after detainee health and provide them with hcv
